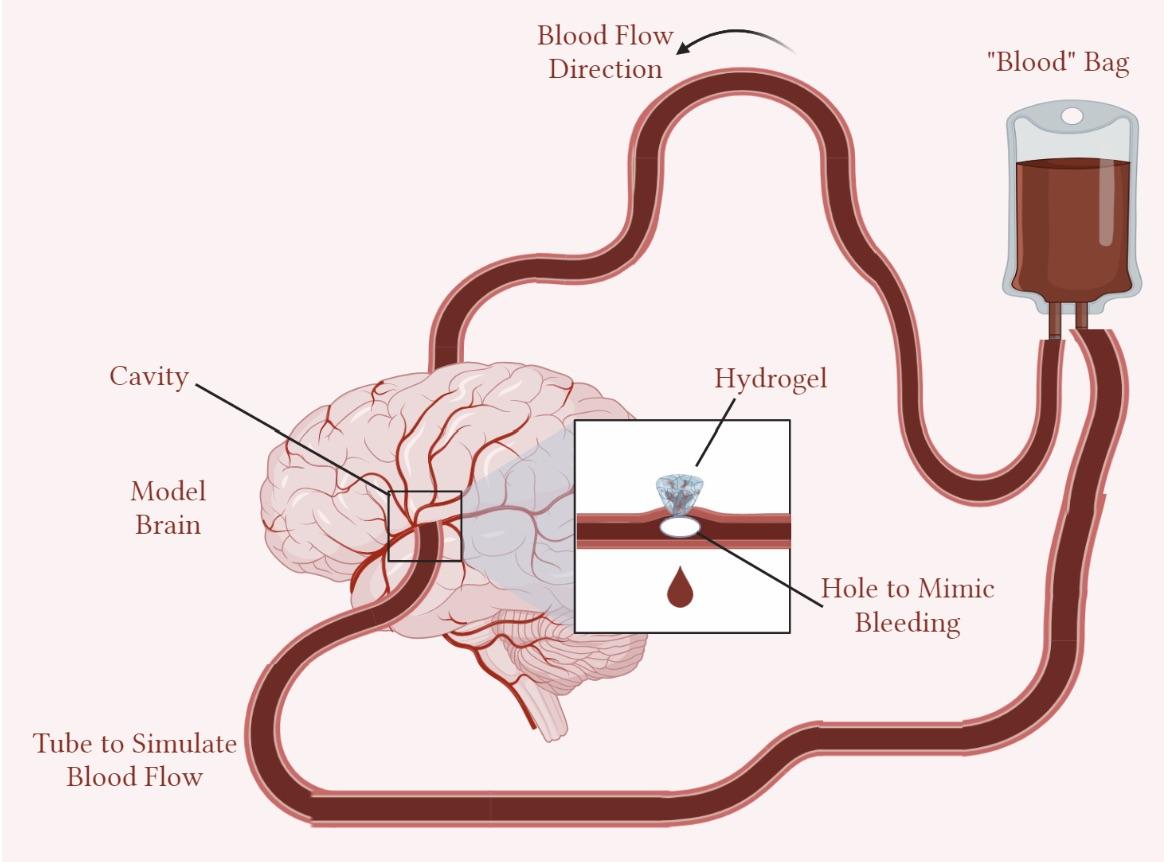
Project Description:
Intracerebral hematomas (ICH) is a condition in which a blood mass or hematoma develops in the brain parenchyma. ICHs are associated with high morbidity and mortality rates. Approximately 40% of patients die within the first month, and only a small percentage (12 - 40%) receive long-term independence. Following ICH evacuation, postoperative rebleeding (PRB) is a common issue that contributes to higher mortality rates. Achieving homeostasis with this type of procedure is challenging; even with strategies like hemostatic injectables, there are still obstacles like capillary bleeding. In this project, we aim to create a biodegradable, injectable hydrogel that exerts pressure to prevent rebleeding. This hydrogel is fabricated from FDA-approved biomaterials, including poly(lactic-co-glycolic acid) and polyethylene glycol. At room temperature, the hydrogel is a liquid that allows easy administration into the patient via a syringe. At average brain temperature (38.5℃), the hydrogel solidifies into its active form and degrades as it interacts with native cerebrospinal fluid steadily. Furthermore, we aim to create a prototype system that simulates the hydrogel delivery in an ICH patient. This system consists of a model brain composed of agar, containing tubing through which blood can flow at a specified rate. Within the brain, there is also a cavity that recapitulates the cavity seen in ICH evacuation, where our hydrogel can be dispensed via a syringe for site management of the ICH, restoring healthy blood flow. Our system effectively simulates the methods for hydrogel delivery and resulting change in blood flow to prove therapeutic success of the intervention. This will provide an easier operation for doctors to treat ICH patients with fewer complications. For this to be accomplished, a series of animal tests will be done to assess the hemostatic efficacy, biocompatibility, and degradation of the animal models induced with ICH. It will move on to extensive human clinical testing, where consent will be obtained from patients who are at high risk of ICH rebleeding or getting consent from health care proxies about using this design. It will benefit the ICH patients and the doctors by providing a less invasive and less complicated solution to their rebleeding issues with their disease. This can be further improved by drug loading the hydrogel, application to other injuries, and developing effective storage mechanisms for the accessibility of low-income countries (LICs).