Project Description:
The goal of this project is the development of a novel device to limit blood sample quality error. To begin, we first wanted to identify what a blood sample quality error was and what caused them. We dove into research regarding the total testing process, techniques applied in blood sampling, examples of blood sample quality errors, data collection for causes of blood sample rejection, and the effect of sample rejection on healthcare costs and patient experience. We found that most errors occur in the pre-analytical phase of blood testing. This is due to the manual labor required for blood sampling. The best way to prevent pre-analytical errors is to improve lab procedure. While this may be a great solution in a low stakes diagnostic setting, unfortunately in the busy healthcare setting this solution is not very feasible. We also identified that some of the main causes for sample rejection were, in the following order of frequency, insufficient sample, clotted sample, incorrect tube, and hemolyzed sample. This inspired us to look further into the cause of these rejections and we came up with three design ideas to address these issues: an automatic detector of improper sample size, a temperature controlled storage casing, and an automatic inverter of sample tubes.
We believe that if a casing were to automatically determine if the sample is over or under filled, the sample could be redrawn faster, which would limit the delay between when the error is discovered and when the sample is redrawn. We also speculate that if samples are held at consistent temperatures, then quality would improve. Additionally, we presume that proper mixing of the sample would result in less error as well. We found that oftentimes the sample tubes are improperly mixed, meaning additives in the tube required for further testing or to prevent clotting are not properly incorporated into the blood. Most tubes must be inverted 5-10 times based on the additives in the tube and eventual test performed. After considering these options, we decided to move forward with the automatic inverter design. We found that there were no designs or patents on the market that provided complete inversions based on the requirements of the sample tube. We identified the tube roller and tube rocker, which gently roll and rock tubes continuously, but do not perform total inversions or even identify the sample tubes appropriately.
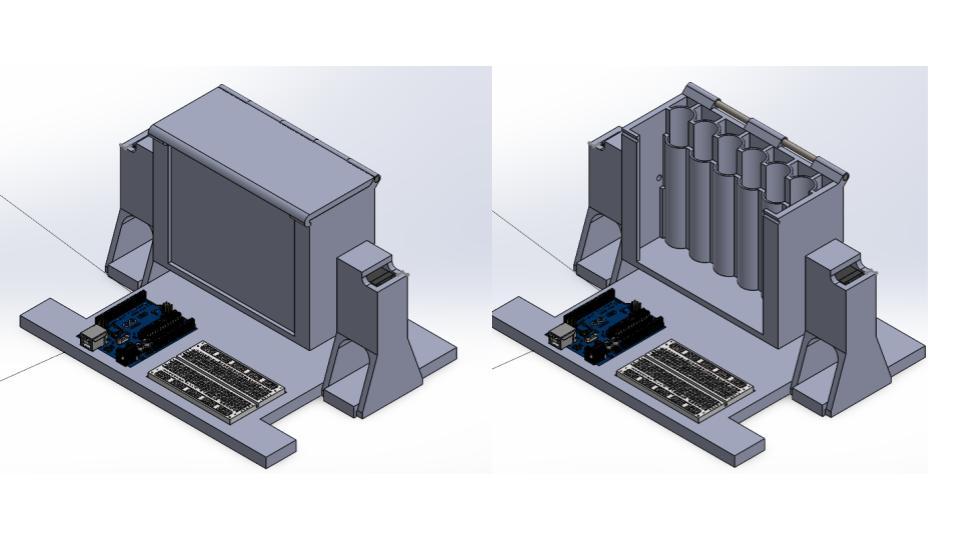
Our design consists of a chassis that houses an electronics component as well as a rotary housing component that houses six BD Vacutainer® blood vials. Photosensors and a MATLAB algorithm detect and sort the samples to calculate the amount of inversions necessary. The proposed design will be automatic, user-friendly, and accurate. We also began planning our experiments to test the effectiveness of our device. These experiments will utilize animal blood and a variety of tubes to see if the device limits sample quality error. We believe the device will reduce hospital costs and improve patient experience, while easing the workload of healthcare workers everywhere.
Advisor/Instructor:
Alisa Morss Clyne, PhDSponsor:
BDTeam Members:
| Dalton Hass | Bioengineering |
| Aidan Kirby | Bioengineering |
| Emilia Pedreros | Bioengineering |
| Abby Rosenberg | Bioengineering |
| Nitya Venkatiahgari | Bioengineering |